Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Matsuda, Shohei; Yokoyama, Keiichi; Yaita, Tsuyoshi; Kobayashi, Toru; Kaneta, Yui; Simonnet, M.; Sekiguchi, Tetsuhiro; Honda, Mitsunori; Shimojo, Kojiro; Doi, Reisuke; et al.
Science Advances (Internet), 8(20), p.eabn1991_1 - eabn1991_11, 2022/05
Times Cited Count:6 Percentile:55.31(Multidisciplinary Sciences)no abstracts in English
 -HF mixture; Kinetics and mechanism
-HF mixture; Kinetics and mechanismSimonnet, M.; Barr , N.*; Drot, R.*; Le Naour, C.*; Sladkov, V.*; Delpech, S.*
, N.*; Drot, R.*; Le Naour, C.*; Sladkov, V.*; Delpech, S.*
Radiochimica Acta, 107(4), p.289 - 297, 2019/04
Times Cited Count:3 Percentile:21.22(Chemistry, Inorganic & Nuclear)Mukai, Yasunobu; Nakamichi, Hideo; Kobayashi, Daisuke; Nishimura, Kazuaki; Fujisaku, Sakae; Tanaka, Hideki; Isomae, Hidemi; Nakamura, Hironobu; Kurita, Tsutomu; Iida, Masayoshi*; et al.
Proceedings of 2017 International Congress on Advances in Nuclear Power Plants (ICAPP 2017) (CD-ROM), 8 Pages, 2017/04
TRP has stored the plutonium in solution state for long-term since the last PCDF operation in 2007 was finished. After the great east Japan earthquake in 2011, JAEA had investigated the risk against potential hazard of these solutions which might lead to make hydrogen explosion and/or boiling of the solution accidents with the release of radioactive materials to the public when blackout. To reduce the risk for storing Pu solution (about 640 kg Pu), JAEA planned to perform the process operation for the solidification and stabilization of the solution by converted into MOX powder at PCDF in 2013. In order to perform PCDF operation without adaption of new safety regulation, JAEA conducted several safety measures such as emergency safety countermeasures, necessary security and safeguards (3S) measures with understanding of NRA. As a result, the PCDF operation had stared on 28th April, 2014, and successfully completed to convert MOX powder on 3rd August, 2016 for about 2 years as planned.
Kobayashi, Fuyumi; Sumiya, Masato; Kida, Takashi; Kokusen, Junya; Uchida, Shoji; Kaminaga, Jota; Oki, Keiichi; Fukaya, Hiroyuki; Sono, Hiroki
JAEA-Technology 2016-025, 42 Pages, 2016/11
A preliminary test on MOX fuel dissolution for the STACY critical experiments had been conducted in 2000 through 2003 at Nuclear Science Research Institute of JAEA. Accordingly, the uranyl / plutonium nitrate solution should be reconverted into oxide powder to store the fuel for a long period. For this storage, the moisture content in the oxide powder should be controlled from the viewpoint of criticality safety. The stabilization of uranium / plutonium solution was carried out under a precipitation process using ammonia or oxalic acid solution, and a calcination process using a sintering furnace. As a result of the stabilization operation, recovery rate was 95.6% for uranium and 95.0% for plutonium. Further, the recovered oxide powder was calcined again in nitrogen atmosphere and sealed immediately with a plastic bag to keep its moisture content low and to prevent from reabsorbing atmospheric moisture.
Segawa, Tomoomi; Fukasawa, Tomonori*; Yamada, Yoshikazu; Suzuki, Masahiro; Yoshida, Hideto*; Fukui, Kunihiro*
Proceedings of Asian Pacific Confederation of Chemical Engineering 2015 (APCChE 2015), 8 Pages, 2015/09
A mixed solution of uranyl nitrate and plutonium nitrate is converted to MOX raw powder by the microwave heating de-nitration method in nuclear reprocessing. Copper oxide synthesized by heating de-nitration was used as a model for the de-nitration process. The microwave heating method (MW) and infrared heating method (IR) were used, and how they and their heating rate influence the obtained particle morphology and size were investigated. The particles obtained by the MW and IR were sufficiently similar in the surface morphology and the mass median diameter was decreased by the increased heating rate. The mass median diameters by the MW were the heating rate and smaller than those obtained by IR. The particle size distribution of the particle obtained by the MW was broader than that by the IR. The relationship of the temperature distribution and particle size distribution by the MW was discussed by the numerical simulation.
Irisawa, Keita; Komatsuzaki, Toshio; Kawato, Yoshimi; Sakakibara, Tetsuro; Nakazawa, Osamu; Meguro, Yoshihiro
JAEA-Technology 2015-008, 28 Pages, 2015/03
In JAEA, 16,671 drums of intermediate-radioactive bituminized waste products (BWPs) have been stored in asphalt solidification storages. As a way of reduction of uncertainty in assessment of disposal of the BWPs, a processing technique of separation of nitrate salts from the BWP by means of an aqueous leaching method was studied. As elemental techniques for the denitration process, (1) crushing techniques of a BWP and (2) denitration techniques for the crushed BWP by the aqueous leaching method were investigated. In order to promote leaching amounts of nitrates, the BWP was crushed, and the grain size distribution was investigated by sieving. Moreover, leaching behaviors of nitrate, nitrite and elements as radionuclides including in the BWP were investigated.
Abe, Hitoshi; Masaki, Tomoo; Amano, Yuki; Uchiyama, Gunzo
JAEA-Research 2014-022, 12 Pages, 2014/11
To contribute safety evaluation of boiling and drying accident of high active liquid waste (HALW) in fuel reprocessing plant, release behavior of Ru, which was considered as an important nuclide for evaluating public dose from the volatile viewpoint, has been investigated. It has been reported that release of Ru becomes conspicuously after HALW is dried up. In this work, to grasp the release behavior of Ru, release ratio of Ru with thermal decomposition of Ru nitrate, which would be in the dried HALW, was measured and release rate constant of Ru from the nitrate was estimated. It was found that the calculation result of release rate of Ru from the nitrate with rise of temperature by using the constant could well simulate the result acquired from the beaker-scale experiment.
Kokusen, Junya; Seki, Masakazu; Abe, Masayuki; Nakazaki, Masato; Kida, Takashi; Umeda, Miki; Kihara, Takehiro; Sugikawa, Susumu
JAERI-Tech 2005-004, 53 Pages, 2005/03
This report presents operating records of dissolution of uranium dioxide and concentration of uranyl nitrate solution and acid removal, which have been performed from 1994 through 2003, for the purpose of feeding 10% and 6% enriched uranyl nitrate solution fuel to Static Experimental Critical Facility(STACY) and Transient Experimental Critical Facility(TRACY) in Nuclear Fuel Safety Engineering Facility(NUCEF).
 -dodecane
-dodecaneApichaibukol, A.; Sasaki, Yuji; Morita, Yasuji
Solvent Extraction and Ion Exchange, 22(6), p.997 - 1011, 2004/12
Times Cited Count:41 Percentile:71.39(Chemistry, Multidisciplinary)no abstracts in English
Watanabe, Shoichi; Yamamoto, Toshihiro; Miyoshi, Yoshinori
Transactions of the American Nuclear Society, 91, p.431 - 432, 2004/11
Temperature effect is a main factor which affects the transient characteristics at a criticality accident. A series of reactivity effects due to changes in fuel temperatures were measured for two kinds of STACY heterogeneous lattice configurations. The core was composed of LWR-type fuel rod array and low-enriched uranyl-nitrate-solution concerning the dissolver of the reprocessing facility for LWR spent fuel. The critical solution heights at various solution temperatures were measured. From the change of the critical water height with fuel temperature, the reactivity effect was evaluated by a critical-solution-level worth method. The temperature effect was also calculated by using SRAC and the transport calculation code TWODANT. The experimental value was estimated to be -2.0 cent/ C for the case "2.1cm-pitch", and -2.5 cent/
C for the case "2.1cm-pitch", and -2.5 cent/ C for the case "1.5cm-pitch". The calculated results gave agreement with the experiments within
C for the case "1.5cm-pitch". The calculated results gave agreement with the experiments within  10%.
10%.
Kida, Takashi; Sugikawa, Susumu
JAERI-Tech 2004-019, 30 Pages, 2004/03
It is known that hydrazine nitrate used in nuclear fuel reprocessing plants is an unstable substance thermochemically like hydroxylamine nitrate. In order to take the basic data regarding the reaction of hydrazine nitrate with nitric acid, initiation temperatures and heats of this reaction, effect of impurity on initiation temperature and self-accelerating reaction when it holds at constant temperature for a long time were measured by the pressure vessel type reaction calorimeter etc. In this paper, the experimental data and evaluation of the safe handling of hydrazine nitrate in nuclear fuel reprocessing plants are described.
Haga, Takahisa*; Gunji, Kazuhiko; Fukaya, Hiroyuki; Sonoda, Takashi; Sakazume, Yoshinori; Sakai, Yutaka; Niitsuma, Yasushi; Togashi, Yoshihiro; Miyauchi, Masakatsu; Sato, Takeshi; et al.
JAERI-Tech 2004-005, 54 Pages, 2004/02
Criticality experiments using uranyl nitrate solution fuel are being conducted at STACY (the Static Experiment Critical Facility) and TRACY (the Transient Experiment Critical Facility) in NUCEF (the Nuclear Fuel Cycle Safety Engineering Research Facility). Chemical analyses of the solution have been carried out to take necessary data for criticality experiments, for treatment and control of the fuel, and for safeguards purpose at the analytical laboratory placed in NUCEF. About 300 samples are analyzed annually that provide various kinds of data, such as uranium concentration, isolation acid concentration, uranium isotopic composition, concentration of fission product (FP) nuclides, tri-butyl phosphoric acid (TBP) concentration, impurities in the solution fuel and so on. This report summarizes the analytical methods and quality management of the analysis for uranyl nitrate solution relating to the criticality experiments.
Mineo, Hideaki; Goto, Minoru; Iizuka, Masaru*; Fujisaki, Susumu; Hagiya, Hiromichi*; Uchiyama, Gunzo
Separation Science and Technology, 38(9), p.1981 - 2001, 2003/05
Times Cited Count:22 Percentile:63.65(Chemistry, Multidisciplinary)no abstracts in English
Tonoike, Kotaro; Miyoshi, Yoshinori; Okubo, Kiyoshi
Journal of Nuclear Science and Technology, 40(4), p.238 - 245, 2003/04
Times Cited Count:2 Percentile:18.9(Nuclear Science & Technology)The reactivity effect of neutron interaction between two identical units containing low enriched (10%  enrichment) uranyl nitrate solution was measured in the STACY. The unit has 350mm of thickness and 690mm of width and distance between those two units was adjustable from 0mm to 1450mm. Condition of the solution was about 290gU/L in uranium concentration, about 0.8N in free nitric acid molarity, 24
enrichment) uranyl nitrate solution was measured in the STACY. The unit has 350mm of thickness and 690mm of width and distance between those two units was adjustable from 0mm to 1450mm. Condition of the solution was about 290gU/L in uranium concentration, about 0.8N in free nitric acid molarity, 24 27
27 C in temperature and about 1.4g/cm
C in temperature and about 1.4g/cm in solution density. The reactivity effect was estimated from variation of critical solution level from 495mm to 763mm depending on the core distance. The reactivity effect was also evaluated by the solid angle method and a computational method using the continuous energy Monte Carlo code MCNP-4C and the nuclear data library JENDL3.2. Comparison of those estimations is presented.
in solution density. The reactivity effect was estimated from variation of critical solution level from 495mm to 763mm depending on the core distance. The reactivity effect was also evaluated by the solid angle method and a computational method using the continuous energy Monte Carlo code MCNP-4C and the nuclear data library JENDL3.2. Comparison of those estimations is presented.
Umeda, Miki; Nakazaki, Masato; Kida, Takashi; Sato, Kenji; Kato, Tadahito; Kihara, Takehiro; Sugikawa, Susumu
JAERI-Tech 2003-024, 23 Pages, 2003/03
MOX dissolution with silver mediated electrolytic oxidation method is planned for the preparation of plutonium nitrate solution to be used for criticality safety experiments at Nuclear Fuel Cycle Safety Engineering Research Facility (NUCEF). Silver mediated electrolytic oxidation method uses the strong oxidisation ability of Ag(II) ion. This method is thought to be effective for the dissolution of MOX, which is difficult to be dissolved with nitric acid.In this paper, the results of experiments on dissolution with 100 g of MOX are described. It was confirmed by the results that the MOX powder to be used at NUCEF was completely dissolved by silver mediated electrolytic oxidation method and that Pu(VI) ion in the obtained solution was reduced to tetravalent by means of NO purging.
purging.
Mitamura, Hisayoshi
JAERI-Review 2003-007, 54 Pages, 2003/03
The inevitable increases of food production and energy consumption with an increase in world population become main causes of an increase of nitrate load to the environment. Although nitrogen is essential for the growth of animal and plant as a constituent element of protein, excessive nitrate load to the environment contaminates groundwater resources used as drinking water and leads to seriously adverse effects on the health of man and livestock. In order to clarify the problem of nitrate contamination of groundwater and search a new trend of technology development from the viewpoint of environmental remediation and protection, the present paper has reviewed adverse effects of nitrate on human health, the actual state of nitrogen cycle, several kinds of nitrate sources, treatment methods for reducing nitrate level, etc.
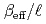 measurement on low enriched uranyl nitrate solution with single unit cores (600
measurement on low enriched uranyl nitrate solution with single unit cores (600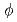 , 280T, 800
, 280T, 800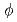 ) of STACY
) of STACYTonoike, Kotaro; Miyoshi, Yoshinori; Kikuchi, Tsukasa*; Yamamoto, Toshihiro
Journal of Nuclear Science and Technology, 39(11), p.1227 - 1236, 2002/11
Times Cited Count:21 Percentile:77.32(Nuclear Science & Technology)Kinetic parameter 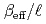 of low enriched uranyl nitrate solution was measured by the pulsed neutron source method in the STACY. This measurement was repeated systematically over several uranium concentrations from 193.7 gU/
of low enriched uranyl nitrate solution was measured by the pulsed neutron source method in the STACY. This measurement was repeated systematically over several uranium concentrations from 193.7 gU/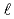 to 432.1 gU/
to 432.1 gU/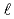 . Used core tanks were two cylindrical tanks whose diameters are 600 mm and 800 mm and one slab tank which has 280 mm thickness and 700 mm width. In this report, experimental data such as solution conditions, critical solution level for each solution condition, subcritical solution levels where measurements were conducted, measured decay time constants of prompt neutron and extrapolated
. Used core tanks were two cylindrical tanks whose diameters are 600 mm and 800 mm and one slab tank which has 280 mm thickness and 700 mm width. In this report, experimental data such as solution conditions, critical solution level for each solution condition, subcritical solution levels where measurements were conducted, measured decay time constants of prompt neutron and extrapolated 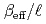 values are described as well as basic principle of the pulsed neutron source method.
values are described as well as basic principle of the pulsed neutron source method. 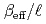 values were evaluated also by computation with the diffusion code CITATION in SRAC and the nuclear data library JENDL 3.2. Both experimental and computational
values were evaluated also by computation with the diffusion code CITATION in SRAC and the nuclear data library JENDL 3.2. Both experimental and computational 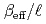 values show good agreement.
values show good agreement.
Aizawa, Eiju; Ogawa, Kazuhiko; Sakuraba, Koichi; Tsukamoto, Michio; Sugawara, Susumu; Takeuchi, Masaki*; Miyauchi, Masakatsu; Yanagisawa, Hiroshi; Ono, Akio
JAERI-Tech 2002-031, 120 Pages, 2002/03
TRACY (Transient Experiment Critical Facility) in NUCEF (Nuclear Safety Research Facility) is the pulse-type critical facility using uranyl nitrate solution which can carry out various supercritical experiments changing reactivity addition up to 3$.TRACY achieved its first criticality on 20th December 1995,and transient operations have been conducted Since1996.This report summarizes the operation data of 176 experiments from the first criticality to FY2000.
Mineo, Hideaki; Goto, Minoru; Iizuka, Masaru*; Fujisaki, Susumu; Uchiyama, Gunzo
Journal of Nuclear Science and Technology, 39(3), p.241 - 247, 2002/03
Times Cited Count:26 Percentile:82.29(Nuclear Science & Technology)no abstracts in English
Terada, Hiroaki; Ueda, Hiromasa*; Wang, Z.*
Atmospheric Environment, 36(3), p.503 - 509, 2002/01
Times Cited Count:49 Percentile:70.13(Environmental Sciences)Acid rain and its neutralization by yellow-sand in East Asia was investigated numerically by an Air Quality Prediction Modeling System (AQPMS). AQPMS consists of advection, diffusion, dry and wet deposition, gas- and liquid-phase chemistry. A new deflation module of the yellow-sand was designed to provide explicit information on the dust loading, and linked to the AQPMS. For model validation, the predicted pH values and sulfate- and nitrate-ion levels of precipitation, together with the surface concentrations of gaseous pollutants, were compared with measured values at atmospheric monitoring stations, and a reasonable agreement was obtained. Firstly, trend of the acid rain in East Asia due to the rapid increase of Chinese pollutants emission was investigated, and a remarkably rapid increase of acid rain area was predicted in the period from 1985 to 1995. Secondly, the simulation results of April 1995 exhibited a strong neutralization of the precipitation by the yellow-sand.